“So long” to the Limits of HER2-Targeted Therapy!
Working in oncology can be tough. Not only do we dedicate our careers to increasing education and awareness for HCPs, patients, and caregivers of life-changing and life-saving drugs, but we’re often impacted by the disease personally. So, not surprisingly, scientific advancements both small and large make us feel something extraordinary.
On Sunday, along with the rest of Hall B1, we felt that something. For the many of us who attend ASCO annually, we can’t wait for the plenary session introduction “we’re looking forward to sharing multiple practice-changing abstracts.”
Dr. Modi received a standing ovation after she presented the results of DESTINY-Breast04 evaluating trastuzumab deruxtecan vs chemotherapy in patients with advanced, HER2-low breast cancer—a testimony to the expected impact of these data on practice.
About half of all patients with advanced breast cancer can be classified as HER2-low, a new categorization that will completely change the way we think about advanced breast cancer treatment. In these patients, who were formerly scattered across HR+/HER2- and triple negative designations, trastuzumab deruxtecan demonstrated significant and unprecedented improvements in PFS and OS - 9.9 mo mPFS and 23.4 mo mOS in all patients - irrespective of hormone receptor positivity. The magnitude of benefit with trastuzumab deruxtecan held across categories of HER2 IHC intensity and prior CDK4/6 inhibitor treatment. These data will usher in a new framework for how we approach breast cancer treatment and set a new benchmark for what we can expect for our patients.
Science has successfully challenged what we thought were the limits of HER2-targeted therapy, with the result of new hope of a significantly extended life for many patients with advanced breast cancer. Tweets from attendees far and wide express hope, excitement, and reflection on how far science has come since Herceptin made it possible to target HER2 24 years ago. We can’t wait to see trastuzumab deruxtecan become a new standard of care.
ASK US WHAT BGB CAN DO RELATED TO:
Defining New Patient Populations | Novel Clinical Trial Design | Evidence Generation Strategy | Market-Shaping Strategy | Scientific Lexicon | Branded Campaign Development | Launch Excellence
Innovation in the Established
As with many tumor types, over the last years, there have been many exciting advancements in prostate cancer. Patients are living longer and with better quality of life. We typically see buzz around new mechanisms of action (MOAs) like 177Lu-PSMA-617, PARP inhibitors, and CAR-Ts. While new mechanisms are exciting to treaters, media, and investors; we can’t overlook the transformative advancements within classes we’ve relied upon for years.
Long considered a cornerstone of treatment for advanced prostate cancer, androgen deprivation therapy is one established category that continues to innovate. First approved in 2019 for non-metastatic castration-resistant prostate cancer, darolutamide (NUBEQA) is an oral androgen receptor inhibitor with a solid clinical profile. Earlier this year and at ASCO 2022, we saw promising data from the phase 3 ARASENS trial, which will likely lead to FDA approval and a new standard of care for metastatic hormone-sensitive prostate cancer. Enzelutamide (Xtandi), another androgen receptor inhibitor that was first approved in 2012, also continues to generate new and exciting data. At ASCO 2022, we saw a follow-up of the phase 3 ENZAMET trial, confirming a survival benefit beyond 5 years in metastatic hormone-sensitive prostate cancer. While these therapies have been approved for years, they continue to push the envelope with new data; now paired with years of experience and trust.
How can established classes generate buzz and ensure success when they are competing against novel MOAs? To stand out amongst the crowd and ensure healthcare professions recognize the innovation happening within these existing classes, memorable, on-point messaging is critical. Advancements across the prostate category are what we’re here for – we travel to ASCO annually for the latest data on the latest drugs. This year, based on what we’ve seen in prostate, it’s important to note that the established continue to impress and we’re excited about how the landscape has evolved for these patients, creating new expectations for standard of care.
ASK US WHAT BGB CAN DO RELATED TO:
Differentiated Messaging | Scenario Planning | Market-Shaping Strategy | Scientific Lexicon | Branded Campaign Development | Launch Excellence
Welcome To the Real World: Reflecting Practice in Clinical Trials
While the FDA provides guidance around trials including study populations reflecting the intended population, clinicians have long noted that the clinical characteristics of patients studied do not always reflect the patients that come through their practice.
We are excited to see this start to change. “Including the Excluded: Advancing Care for All Patients With Lung Cancer” addressed this concern, as it relates to CNS metastases, performance status, and age. In this session, two abstracts discussed studies in patients with NSCLC and active, untreated CNS metastases (abstracts LBA9009 and9010), and another abstract discussed a study in patients with NSCLC who had an ECOG PS of 2 or were elderly (≥70) (abstract 9011).
Inclusion matters! We see brain metastases as common in patients with melanoma and lung cancers (~15%-25% at diagnosis) and the percentages can increase quite a bit when we look at association with specific mutations. And the risk doesn’t stop at diagnosis – some mutations are associated with a rate of developing brain metastases of over 50% over the course of the disease.
In two abstracts, systemic therapies showed intracranial activity and prolonged time until the need for local therapy such as radiotherapy. The first looked at patients with advanced NSCLC and untreated CNS metastases treated with adagrasib in the KRYSTAL-1 trial, in which 32% of patients achieved intracranial ORR (including 3 complete responses), mDOR was not reached, and intracranial mPFS was 4.2 months. The second looked at patients with advanced non-squamous NSCLC and untreated CNS metastases treated with atezolizumab + carboplatin/pemetrexed, in which intracranial ORR was 42.5% (including 5 complete responses) and intracranial mPFS was 6.9 months. No differences in intracranial responses were seen between patients who were PD-L1 positive and PD-L1 negative. The median time to brain radiotherapy was 10.9 months, and EFS, defined as the time from study inclusion to brain radiotherapy or death, was 10.9 months. Given the poor prognosis of patients with these disease characteristics (median OS for patients with untreated CNS metastases is ~5 months), these data demonstrate the importance of including these patient populations in clinical trials to ascertain the potential benefits of novel anticancer therapies.
In a guidance to industry, the FDA has noted that, despite clinical trials not having an upper age limit for exclusion, adults ≥75 years old continue to be underrepresented. Similarly, patients with ECOG PS ≥2 are often excluded from clinical trials, including those studying immunotherapy agents. Data from a phase 3 study of nivolumab + ipilimumab vs carboplatin in the first-line treatment of patients with advanced NSCLC and ECOG PS 2 or ≥70 years demonstrated a detrimental impact on OS in patients with PS 2 at a preplanned interim analysis, which led the DMC to recommend stopping enrollment. For patients ≥70 and PS 0/1, there was a statistically significant improvement in mOS with nivolumab + ipilimumab vs platinum doublet chemotherapy (22.6 months vs 11.8 months). These data show that trials for elderly patients and patients with PS 2 are feasible and needed.
As clinicians and regulators push for inclusion of often excluded or underrepresented populations in clinical trials, the oncology field can start to have a more complete picture of the efficacy and safety of anticancer treatment regimens across real-world patient populations.
ASK US WHAT BGB CAN DO RELATED TO:
Real World Evidence Strategy | Thought leader Engagement | Value Proposition Development | Novel Clinical Trial Design | HCP Insight Mining | Medical Education
A Shifting DLBCL Treatment Landscape
Before the 1980s, standard therapy for localized DLBCL was radiation. The introduction of chemotherapy, specifically CHOP followed by R-CHOP, established a new standard of care that has remained largely unchanged for the past 40 years. Relapse rates of up to 40% have left a need in the treatment algorithm for effective 2L+ therapies as well as better frontline control of disease. Recent years have seen the entry of game-changing therapies across different modalities and continuing the excitement we experienced at ASH 2021, ASCO 2022 showed us just how fast this field is developing with some very exciting data.
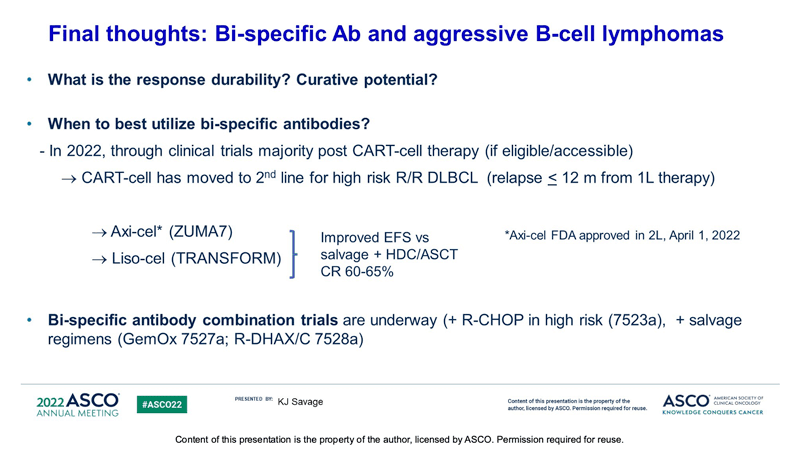
One of ASCO 2022’s first heme presentations showed us expanded pivotal phase 2 trial data of glofitamab (Genentech’s CD20xCD3 bispecific antibody) in patients with R/R DLBCL. Data showed positive responses with fixed-duration glofitamab in patients who were heavily pretreated and highly refractory. 39.4% of patients had a complete response (51.6% ORR) and the median duration of response was 18.4mo. Reported CRS was mostly low grade and usually occurred in the first cycle. Primary results of subQ epcoritamab (Genmab’s CD20xCD3 bispecific) dose expansion in patients with R/R DLBCL is set to be presented as a late breaking presidential abstract at EHA next week. The abstract reports a similarly highly-refractory patient population to the glofitamab trial (double/triple hit, median 3 prior LOT) and an ORR rate of 63% and CR of 39%, data which are largely consistent in both CART-naïve and prior CAR-T patients. Given these data, and potential approvals this year, it will be interesting to see where bispecific antibodies net in the treatment paradigm. Will they be prioritized ahead of cell therapies in consideration of their “off the shelf” nature? Or will HCPs elect to save bispecific therapy as a post CAR-T option, particularly in patients who may lose CD19 antigen expression?
ASCO DLBCL presentations continued the theme of exploring treatments specifically in high-risk patients. First in frontline: The phase 3 POLARIX study (presented at ASH 2021) demonstrated superior efficacy of polatuzumab vetodin plus R-CHP over R-CHOP for frontline treatment of large B-cell lymphomas. This combination has now been approved by the European Commission and is expected to be approved in the US later this summer. However, only 6.7% of enrolled patients in the POLARIX study had high-grade B-cell lymphoma. Preliminary data presented at ASCO 2022 in a small patient population (N=18) showed that polatuzumab vedotin can be safely incorporated into a frontline chemotherapy treatment regimen in patients with high-grade, aggressive disease. Similarly, data presented for the EPCORE NHL-2 trial showed that the addition of epcoritamab to standard of care R-CHOP in first line treatment of high risk DLBCL yielded clinically meaningful responses (ORR=100%; 77% CMR, 23% PMR) and manageable safety. In the relapsed/refractory setting, sub-group analyses were presented from the observational RE-MIND study which compared patient outcomes from the phase 2 L-MIND study (examining the effects of tafasitamab plus lenalidomide in R/R DLBCL patients) to closely matched real-world cohorts. Data showed that in patients with stem cell transplant-ineligible high-risk DLBCL, treatment with tafasitamab plus lenalidomide demonstrated improved OS when compared to systemic therapies (34.1 mo vs 11.6 mo), pola-BR (20.1mo vs 7.2mo), and CD19-directed CAR-T therapy (22.5mo vs 15mo). Although most of these data are preliminary, it is exciting to see these treatments being explored in difficult-to-treat, high-risk patients.
With the introduction of CAR-Ts into 2L possibly replacing the need for “salvage” chemo and ASCT, the potential for the first new frontline therapy in 40 years, and entry of bispecific antibodies into the treatment algorithm, 2022 is poised to be the most significant and paradigm-shifting year the DLBCL field has experienced in a while, one that will hopefully provide patients with more options than ever before.
ASK US WHAT BGB CAN DO RELATED TO:
Belief & Behavior Change | Unbranded Campaign Development | Payer Strategy